Which Statement Best Describes an Ionic Bond
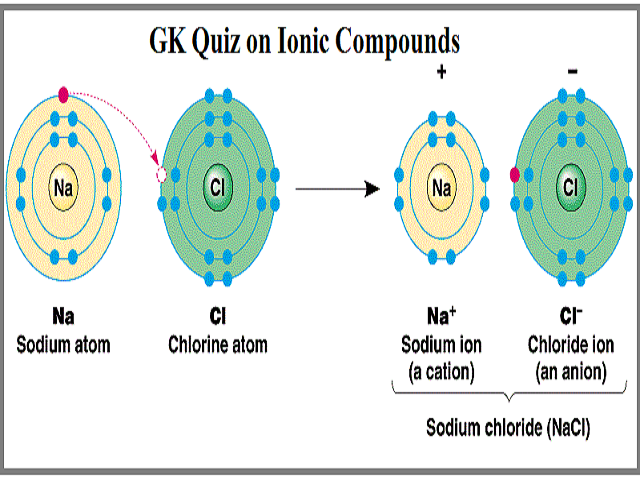
Ionic bonds are formed as a result of complete transfer of electrovalence electrons from one atom to another. The transfer of electrons results in attractive forces between molecules.
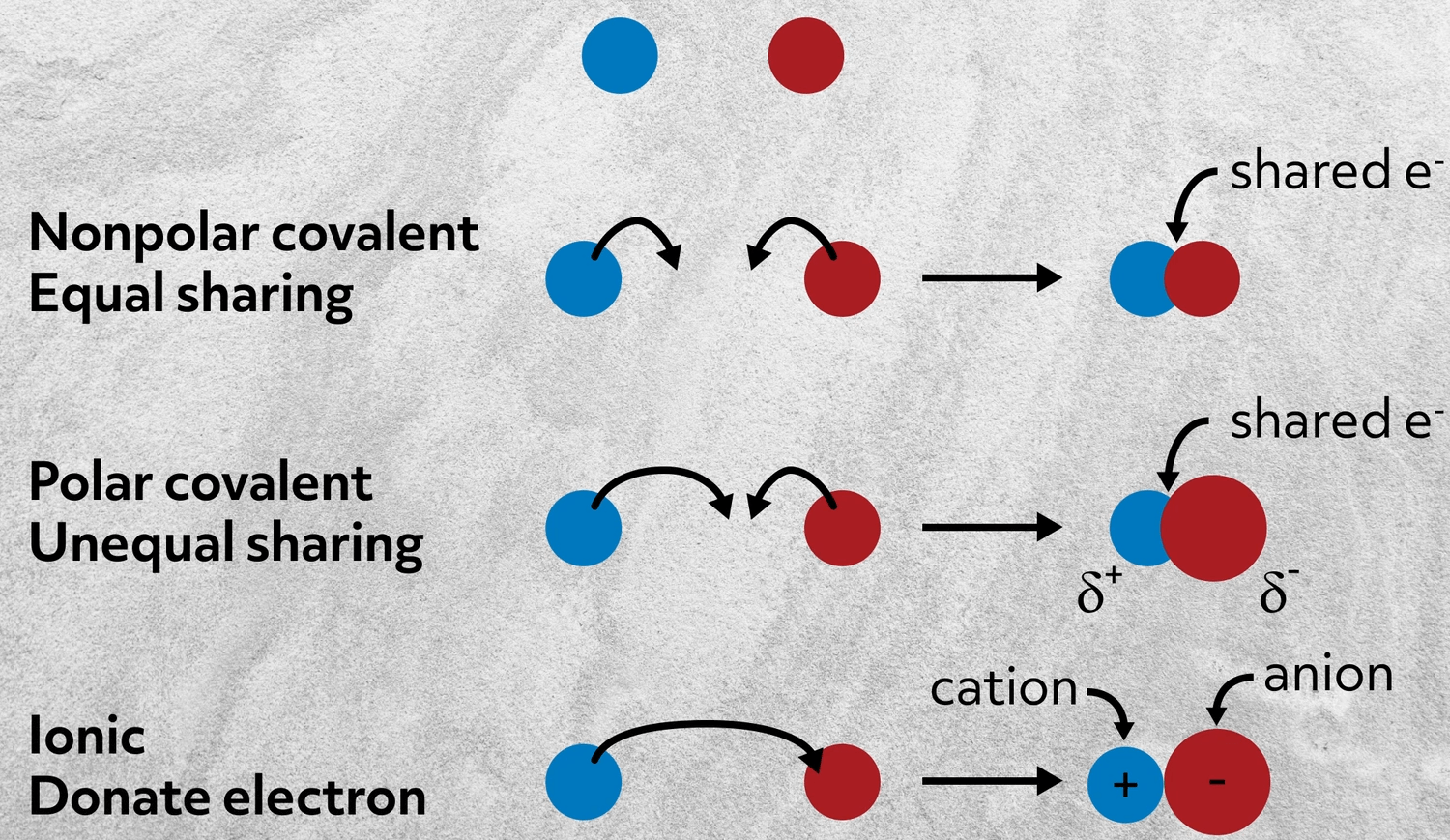
An ionic bond involves a metal that shares electrons with a nonmetal.
. One atom pulls an electron from another atom. An ionic bond involves two nonmetals that share electrons. The sharing of electrons forms strong bonds between ions.
Which statement best describes ionic bonding in lithium fluoride. It is insoluble in water. The statement best describes the Ionic and Covalent bonds is ------ A In ionic bonds electrons are shared while.
The atom that donate the electron become a positively charged ion while the atom that received the atom become a negatively charged ion. The transfer of electrons forms strong bonds between ions. 2 Which is the best description of a molecular compound.
Which statement best describes an ionic bond. An ionic bond involves a metal that transfers one or more electrons to a nonmetal. 4 What does it mean when a compound is molecular.
The question stateswhich statement best describes how an ionic bond forms. It can be easily flattened. The sharing of electrons results in attractive forces between molecules.
A The positive and negative charges of the ions cancel out. B A lithium atom shares on electron with a fluorine atom C An electron is transferred from a lithium atom to a fluorine atom. 1 Which best describes what happens when molecular compounds melt chegg.
Ionic bonds are formed as a result of complete transfer of electrovalence electrons from one atom to another. Some properties of ionic compounds are high melting points. The correct option is A.
The attraction of ions due to the transfer of valence electrons. The statement which best describes an ionic bond is. A chemical bond refers to the forces of attraction that exist between ions crystals atoms or molecules and as such are typically responsible for the formation of new chemical compounds.
Ionic bonds are formed as a result of complete transfer of electrovalence electrons from one atom to another. Ionic Bond is formed due to attraction between Cation and an Anion. Click to see full answer Similarly you may ask what best describes an ionic bond.
It is a type of chemical bond that generates two oppositely charged ions. An ionic bond involves two metals that exchange electrons. The question sayswhich statement best describes how an ionic bond forms.
An ionic bond involves two nonmetals that share electrons. The question sayswhich statement best describes how an ionic bond forms. What statement best describes the properties of ionic compounds.
While an Anion is formed when an atom mainly Non-Metal accepts or gain electron s. In ionic bonds the metal loses electrons to become a positively charged cation whereas the nonmetal accepts those electrons to become a negatively charged anion. Maxonik 38 1 year ago.
An ionic bond involves two metals that exchange electrons. The sharing of electrons results in attractive forces between molecules. Which statement best describes ionic bond.
The correct option is A. It is called an ionic bond because when an element gives up or accepts electrons it gains either a positive or negative charge respectively. Two atoms attain equal electronegativities.
Option A is the correct answer as ionic compounds are made of positively-charged cations and negatively-charged anions. Ionic bonding is the complete transfer of valence electrons between atoms and is a type of chemical bond that generates two oppositely charged ionsSimilarly nonmetals that have close to 8 electrons in its valence shell tend to readily accept electrons to achieve its noble gas. Ionic bonding is the complete transfer of valence electrons between atoms.
An ionic bond involves a metal that transfers one or more electrons to a nonmetal. An ionic bond involves a metal that shares electrons with a nonmetal. Just think of positive and negative charges attracting to one another thats really all an ionic.
Which property best indicates that a compound contains an ionic bond. The transfer of electrons results in attractive forces between molecules. The atom that donate the electron become a positively charged ion while the atom that received the atom become a.
Which statement best describes how an ionic bond forms. An ionic bond involves two nonmetals that share electrons. D An electrostatic attraction exists between lithium ions and fluoride ions.
An ionic bond involves a metal that shares electrons with a nonmetal. It has a low boiling point. The correct option is A.
Which of the following happens when an ionic bond is formed. Which statement explains the relationship between the amount of energy it takes to break a bond and the amount of energy released when the same bond is formed. The transfer of electrons forms strong bonds between ions.
Which statement best describes an ionic bond. Ionic bonds always take place between a metal and nonmetal. An ionic bond involves a metal that transfers one or more electrons to a nonmetal.
The atom that donate the electron become a positively charged ion while the atom that received the atom become a negatively charged ion. The sharing of electrons forms strong bonds between ions. Metals usually give up electrons and nonmetals usually accept electrons.
5 Which of the following statements correctly describes the role of water as a solvent. 3 Do molecular compounds burn easily. Which statement best describes how an ionic bond forms.
The formation of Cation takes place when an atom mainly Metal looses electron s. Which statement best describes ionic compounds. There is not a statement available so it is difficult to answer this.
2Octet rule is ---- A tendency of atoms to react in ways to achieve outer shell of eight valence elections. It conducts electricity when molten.

Ionic Bond Definition Properties Examples Facts Britannica
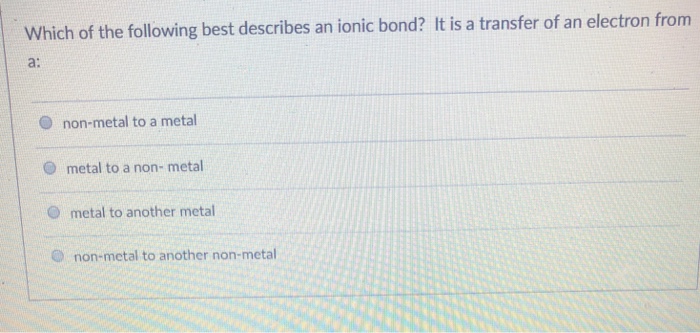
Solved Which Of The Following Best Describes An Ionic Bond Chegg Com

Komentar
Posting Komentar